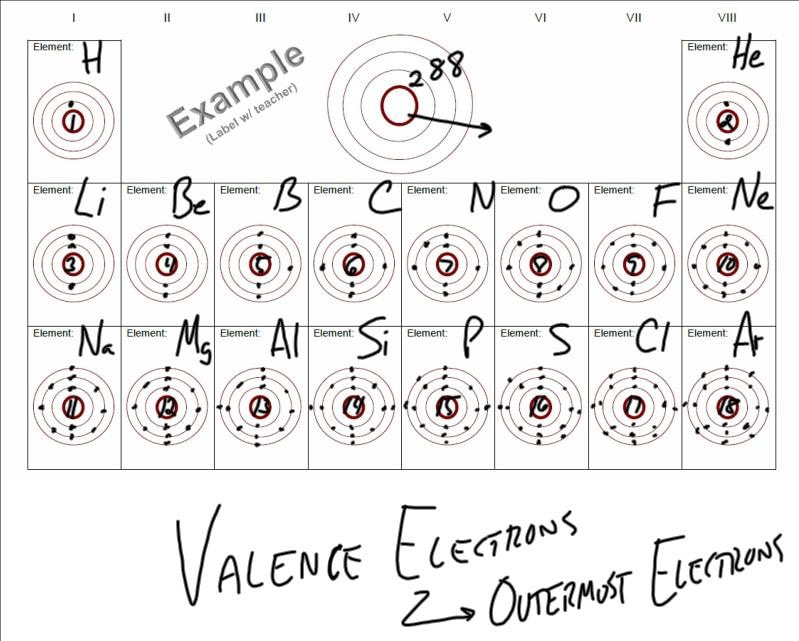
Notice that all elements sharing the same column have the same number of valence electrons*. (e.g. Boron [B] has three valance electrons just like Aluminum [Al] does. Nitrogen [N] has five valance electrons just like Phosphorous [P] does.)
Notice as well that all elements sharing the same row occupy the same number of electron energy levels (e.g. Lithium [Li] has electrons in the first two energy levels just like Flourine [F] does. Magnesium [Mg] has electrons in all three energy levels just like Sulfur [S] does.)
*Although Helium [He] has two valance electrons, it is commonly placed in the far-right column because its outermost energy level is full, just like Neon [Ne] and Argon [Ar].
Notice as well that all elements sharing the same row occupy the same number of electron energy levels (e.g. Lithium [Li] has electrons in the first two energy levels just like Flourine [F] does. Magnesium [Mg] has electrons in all three energy levels just like Sulfur [S] does.)
*Although Helium [He] has two valance electrons, it is commonly placed in the far-right column because its outermost energy level is full, just like Neon [Ne] and Argon [Ar].












 RSS Feed
RSS Feed